- Technical Flexibility and Rigidity Webinar (private)
Week 6 – Exploring cultural techniques in non-human animals: how are flexibility and rigidity expressed at the individual, group, and population level?
This early draft was authored by Sadie Tenpas, Manon Schweinfurth, and Josep Call.
Non-human animal (henceforth animal) culture has been demonstrated across a variety of species and, for some, includes technical traditions. Like human culture, animal culture faces the dilemma of balancing flexible and rigid behaviour. On one hand, a culture must allow for the creation, acquisition, or novel application of cultural behaviours. On the other hand, a culture must ensure a high degree of similarity between individual performances, leading to stable traditions that persist across generations despite disruptive influences. The dominant argument to understand the rigidity of cultural transmission within animals has focused on social learning, with mechanisms such as imitation leading to the highest fidelity of transmission. Thereby animal culture has often been limited to examples explained by social learning alone and has historically excluded behaviours that could be explained by genetic or ecological factors. In this chapter we argue that the effect of these factors, rather than exclude, may assist social learning in explaining cultural differences. To explore these concepts further, we will examine and define flexible and rigid behaviour at the individual level, then expand to the group and population level. We propose a framework that combines social learning theory and the Cultural Attraction Theory to non-human primate cultural techniques, who are our closest living relatives and thus can give insights into human culture. We argue that cultural traits are the product of three poles of attraction, i.e. individual predispositions, social influences and ecological contexts. This new framework can contribute to better understanding the origins, persistence, and eventual demise of cultural techniques.
Introduction
The existence of animal culture, understood as those group typical behaviour patterns that are shared by members and that rely on socially learned and transmitted information (Laland & Hoppitt, 2003), is commonly accepted today. Following over 50 years of debate, we have convincing evidence of cultural behaviour in birds (Aplin, 2019), cetaceans (Day et al. 2001; Allen et al. 2013), and primates (Whiten & van de Waal, 2017), among others (Laland & Evans, 2017; Whiten, 2019). Cultures consist of traditions that are distinct behaviour patterns, shared by at least two members of a group, which persist over time and that new practitioners acquire, in part, through socially aided learning (Fragaszy & Perry, 2003). These traditions can include techniques that produce material change (Lamon et al., 2017). For example, chimpanzees (Pan troglodytes) create and use stick tools during termite fishing (Goodall, 1964). However, for the purpose of this chapter we will extend our definition of techniques beyond cases that produce material changes to include more cultural traditions, such as different manual techniques to perform a certain action. In such, we understand techniques as complex actions whose instrumental function is to produce changes in the environment, both physical and social.
The ability to ratchet up the complexity of cultural traditions, leading to both increased efficiency and productivity, is created by cumulative culture (Tennie et al. 2009; Dean et al. 2014); a phenomenon primarily observed, and by some exclusively described, in humans (Dean et al.2014). Although the learning of many animal species is influenced by the observation of or interaction with another animal or its products (Heyes, 1994), there is minimal evidence of cumulative culture in animals (but see – Sasaki & Biro, 2017). The richest evidence of animal culture comes from one of our closest living relatives, chimpanzees (Marshall-Pescini & Whiten, 2008). Therefore, we will focus primarily on this species even though we will also consider data from other species to shed light onto our own cultural evolution.
Long-term research of chimpanzees has provided us with extensive documentation of cultural traditions, highlighting variation between communities and distinct cultural repertoires. Most famously, Whiten et al. (1999) collected candidate cultural behaviours from seven long-term field sites across Africa. Using a systematic approach, i.e. the method of exclusion or the ethnographic method, candidate behaviours shown only to occur within some communities were determined to be cultural when the variability between groups could not be otherwise explained by ecological of genetic differences, thus leaving social learning as the remaining source of the observed variation (Whiten et al. 1999).
While chimpanzees show a great variety of traditions, there is little evidence that these traditions are frequently updated through additional knowledge or behaviours (e.g. Gruber et al., 2009; but see Biro et al., 2003). This tendency toward stasis is especially striking considering that chimpanzees demonstrate an impressive ability to innovate solutions to novel problems (Bandini & Harrison, 2020). So, while chimpanzee cultures may not reflect the same process or complexity of human cumulative culture, chimpanzee culture still undergoes cultural evolution in which a balance must be struck between the novel creation, variation, or application of cultural behaviours and the high degrees of similarity between individual performances, leading to stable traditions that persist across generations despite potential disruptive influences. Thus, the questions arise, which mechanisms and factors drive chimpanzee cultures toward stasis, which drive them toward change, and how can we understand this in respect to cultural techniques?
To address these questions, we will begin by exploring the relationship between flexibility and rigidity in culture at the individual, group and population level. Using these concepts, we will investigate how flexibility and rigidity in chimpanzee culture are currently understood and how social learning explains them. After highlighting the limitations of the social learning perspective, we will address an alternative theory utilized in the cultural evolutionary literature that may shed insights into factors supporting cultural variation and stability. We propose a framework that combines these two theories to provide a more satisfactory explanation of the occurrence of flexibility and rigidity in chimpanzee techniques.
Defining flexibility and rigidity in primate techniques
To fully capture the concept of cultural evolution, we will illustrate different levels of flexibility and rigidity in primate cultural techniques, i.e. individual, group, and population. Let’s imagine a scenario in which a chimpanzee uses a wooden hammer to crack open nuts. Beginning at the individual level, we define a spectrum with liberalism, describing an individual’s tendency or disposition to change, on one end and conservatism, describing an individual’s tendency or disposition to remain the same, on the other end (Figure 1a). Note that we expect to see variation both within and between individuals along this spectrum. In some cases, driving a change in behaviour is necessity, which we understand to occur when an individual can improve the efficiency of their technique by updating it through modification, variation, or invention of behaviour. By distinguishing the necessity for a change in an individual’s behaviour, or lack thereof, we can further breakdown liberal and conservative behaviour in respect to adaptive decisions. Consider, for example, that an environmental change resulted in increased hardness of the nuts used for extractive foraging such that a wooden hammer was no longer hard enough to crack the nut. Therefore, the liberal individual selects a different hammer, such as a stone to crack the nut. Under this condition, the individual is behaving innovatively, responding to the necessity for change. Alternatively, if we consider an example in which the nutshell remains the same hardness, yet the liberal individual switches hammers anyway, the individual is behaving creatively because it changes its behaviour despite no need for change. When considering the individual behaving conservatively, the same scenarios apply. If the conservative individual maintains their behaviour, but there is need for change, the individual is behaving perseveringly. If the conservative individual maintains their behaviour, and there is no need for change, the individual is behaving consistently.
Broadening to the group level, we introduce social factors guiding behavioural change (or lack of) using the same context and framework as before. First, in defining a spectrum at the group level, we have flexibility, describing a group’s tendency to change a behaviour, on one end and rigidity, describing a group’s tendency to maintain a behaviour, on the other end (Figure 1b). Envisioning the same nut cracking scenario, when a flexible group needs to change their behaviour and does so through successful social learning and transmission, this change can be described as an advancement. When a flexible group does not need to change their behaviour, yet does anyway, this change can be described as a shift. Alternatively, when a rigid group needs to change their behaviour but does not, this lack of change can be described as fixedness. When a rigid group does not need to change their behaviour, and does not, this lack of change can be described as stasis.
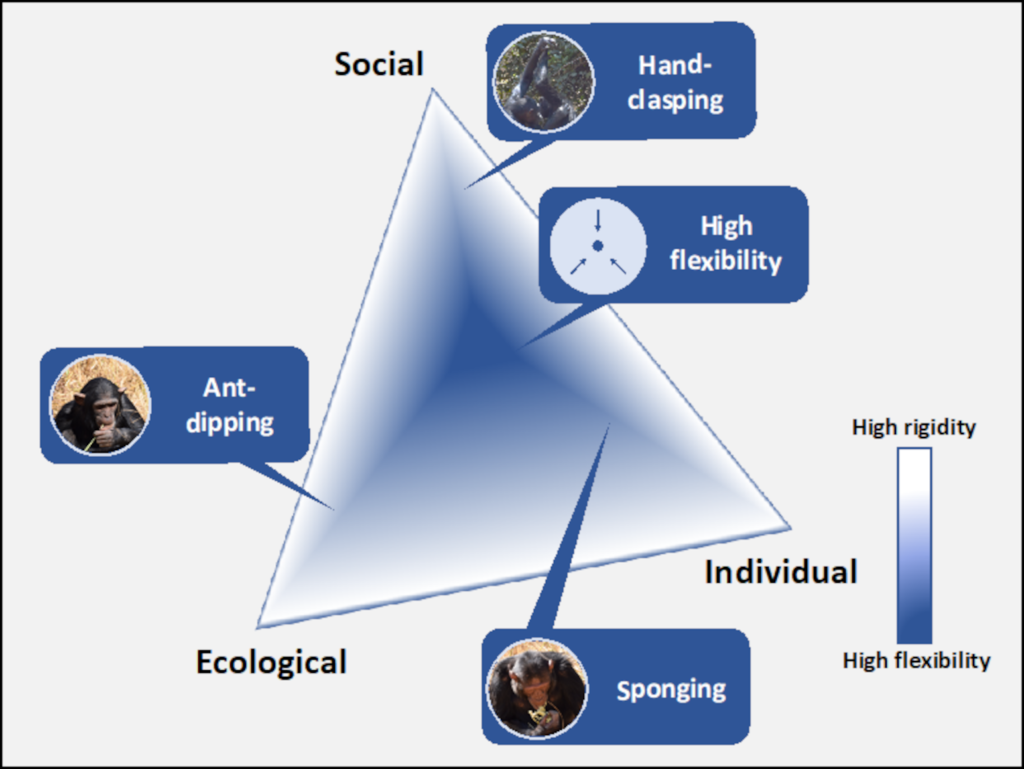
Ant-dipping is a foraging technique in which an individual selects a wand tool, inserts the wand into a nest or near a trail of ants, may move or hold the wand still to collect ants, then removes the ants by either bringing the wand directly to the mouth or by using the opposite hand to remove ants and put into the mouth (Humle, 2011). In contrast to sponging, ant-dipping is only present in some chimpanzee populations and the technique differs in terms of material means and actions (McGrew, 1992; Whiten et al., 1999; Yamakoshi, 2001; Humle & Matsuzawa, 2002 Humle, 2011). Depending on the aggression of the ants present, chimpanzees will select wands of different length and apply different ant gathering techniques to avoid severe bites. Under conditions with high probability for bites, the ecological influence becomes more important. The behaviour is predicted to be more rigid to avoid discomfort. In a setting where ants vary in aggressiveness or show low levels, behaviours are predicted to be more flexible, allowing for more variation. Indeed, chimpanzees from sites with more aggressive ants use longer tools and collect the ants by hand wiping them from the wand whereas those from sites with less aggressive ants were dipped with shorter tools associated with the direct to mouth method (Schöning et al. 2008). Strikingly, the ecology can probably not exclusively explain ant-dipping techniques as chimpanzees from two sites with ants that show the same level of aggression use differently sized wands (Möbius et al. 2008). This suggests that social learning may play an important role in maintaining these variations (Humle, 2011). In combination with social learning, individual experience of developing chimpanzees may further reinforce a group-specific technique as they encounter ant bites. Through combining strong ecological influences with social learning and individual experience, we can imagine that variation of techniques between groups is maintained.
In addition to these two material-based examples, hand-clasping is a social custom that is only present in some groups and varies between groups. Hand-clasping is a social grooming technique where two individuals clasp hands or press wrists or forearms (or other combinations of two) together overhead and groom the other with their free hand (McGrew & Tutin, 1978). Due to the dyadic nature of this grooming technique, it is unlikely that variations of this behaviour would arise out of, or be maintained by, individual dispositions alone. It might have originated by holding arms overhead in a comfortable manner for which branches are needed (McGrew et al. 2001), highlighting an ecological influence. Still, groups kept under the same ecological condition, show different techniques, suggesting that variations are socially determined (McGrew et al. 2001; Nakamura & Uehara, 2004; van Leeuwen et al. 2012). While it is not known exactly which mechanisms support this transmission, we could speculate that high-fidelity mechanisms such as ontogenetic ritualization, in which two individuals shape one another’s behaviour through repeated interaction (Tomasello & Call, 1997), or imitation in combination with individual and ecological influences maintain group-specific variations. In this context, one could imagine that the strong influences of social learning and the dyadic nature of the technique maintain group rigidity, in addition to the appropriate ecological context (i.e. no available branches) and limited individual influence.
In summary, pole strength can limit the variations and inventions an individual can produce and in turn limit how innovations spread through a group. However, when polar influences are less strong or cancel each other out, opportunity for change can arise and be supported both at the individual and group level. Depending on the species, the potential impact each pole exerts over the transmission and performance of techniques may vary. For example, an animal group that does not highly affiliate or cohabitate between group members may not see as strong an influence by the social pole and may not have as many socially transmitted and sustained techniques. Conversely, we can imagine that humans are very strongly influenced by the social pole allowing for the use of mechanisms like teaching and the accumulation of techniques beyond the individual. Further, individual factors may more strongly influence humans allowing for a wide array of stylistic preferences liberally demonstrated. In such, an appropriate balance between the polar influences can provide the opportunity for liberal invention and variation by individuals and flexible, yet stable adoption through social learning by groups, resulting in cultural evolution.
Conclusion
Our multipolar framework combines the insights of cultural attraction and social learning theories to produce a more complete understanding of animal cultural behaviour. We do not view flexibility and rigidity along a single dimension in which individuals or groups behave one way or the other. Rather, we understand the expression of flexible and rigid behaviour as the outcome of the dynamic process created by the unique influence of each pole in a given context. This framework helps us to unify CAT and the social learning perspective, reconciling their limitations; namely, CAT’s de-emphasis on social learning mechanisms and the social learning perspective’s inability to meaningfully incorporate the role of ecology and genetics. We do not believe that these differences should represent mutually exclusive reasoning, but rather that when brought together, offer solutions for understanding one another. In such, we use this combined framework to illuminate that chimpanzee populations are not strictly flexible or rigid, but rather that their flexibility and rigidity arise differentially as an outcome of dynamic polar attraction. Through this lens we understand that the traditions and techniques demonstrated in chimpanzee cultures are a product of combined social, individual, and ecological influences which have allowed for the evolution of the distinct repertoires we observe today.
References
Allen, J., Weinrich, M., Hoppitt, W., & Rendell, L. (2013). Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science, 340(6131), 485-488.
Aplin, L. M. (2019). Culture and cultural evolution in birds: a review of the evidence. Animal Behaviour, 147, 179-187.
Bandini, E., & Harrison, R. A. (2020). Innovation in chimpanzees. Biological Reviews.
Biro, D., Inoue-Nakamura, N., Tonooka, R., Yamakoshi, G., Sousa, C., & Matsuzawa, T. (2003). Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Animal cognition, 6(4), 213-223.
Buttelmann, D., Carpenter, M., Call, J., & Tomasello, M. (2007). Enculturated chimpanzees imitate rationally. Developmental science, 10(4), F31-F38.
Buskell, A. (2017). What are cultural attractors?. Biology & Philosophy, 32(3), 377-394.
Call, J., Carpenter, M. & Tomasello, M. 2005 Copying results and copying actions in the process of social learning: chimpanzees (Pan troglodytes) and human children (Homo sapiens). Animal Cognition 8, 151–163.
Charbonneau, M. (2018). Understanding cultural fidelity. The British Journal for the Philosophy of Science.
Claidière, N., & Sperber, D. (2007). The role of attraction in cultural evolution. Journal of Cognition and Culture, 7(1-2), 89-111.
Claidière, N., & Sperber, D. (2010). Imitation explains the propagation, not the stability of animal culture. Proceedings of the Royal Society B: Biological Sciences, 277(1681), 651-659.
Custance, D. M., Bard, K. A., & Whiten, A. (1995). Can young chimpanzees (Pan troglodytes) imitate arbitrary actions? Hayes & Hayes (1952) revisited. Behaviour, 132(11-12), 837-859.
Day, R. L., Kendal, J. R., & Laland, K. N. (2001). Validating cultural transmission in cetaceans. Behavioral and Brain Sciences, 24(2), 330.
Dean, L. G., Vale, G. L., Laland, K. N., Flynn, E., & Kendal, R. L. (2014). Human cumulative culture: a comparative perspective. Biological Reviews, 89(2), 284-301.
Fragaszy, D. M., & Perry, S. (2003). Towards a biology of traditions. The biology of traditions: models and evidence, 1-32.
Goodall, J. (1964). Tool-using and aimed throwing in a community of free-living chimpanzees. Nature, 201(4926), 1264-1266.
Gruber, T., Muller, M. N., Strimling, P., Wrangham, R., & Zuberbühler, K. (2009). Wild chimpanzees rely on cultural knowledge to solve an experimental honey acquisition task. Current biology, 19(21), 1806-1810.
Heintz C., Claidière N. (2015) Current Darwinism in social science. In: Heams T., Huneman P., Lecointre G., Silberstein M. (eds) Handbook of evolutionary thinking in the sciences. Springer, Dordrecht
Heyes, C. M. (1994). Social learning in animals: categories and mechanisms. Biological Reviews, 69(2), 207-231.
Hobaiter, C., Poisot, T., Zuberbühler, K., Hoppitt, W., & Gruber, T. (2014). Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol, 12(9), e1001960.
Hoppitt, W., & Laland, K. N. (2013). Social learning: an introduction to mechanisms, methods, and models. Princeton University Press.
Horner, V., & Whiten, A. (2005). Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Animal cognition, 8(3), 164-181.
Humle, T., & Matsuzawa, T. (2002). Ant‐dipping among the chimpanzees of Bossou, Guinea, and some comparisons with other sites. American Journal of Primatology: Official Journal of the American Society of Primatologists, 58(3), 133-148.
Humle, T. (2011). Ant-dipping: how ants have shed light on culture. In The chimpanzees of Bossou and Nimba (pp. 97-105). Springer, Tokyo.
Kendal, J., Giraldeau, L. A., & Laland, K. (2009). The evolution of social learning rules: payoff-biased and frequency-dependent biased transmission. Journal of theoretical biology, 260(2), 210-219.
Kendal, R., Hopper, L. M., Whiten, A., Brosnan, S. F., Lambeth, S. P., Schapiro, S. J., & Hoppitt, W. (2015). Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evolution and Human Behavior, 36(1), 65-72.
Kendal, R. L., Boogert, N. J., Rendell, L., Laland, K. N., Webster, M., & Jones, P. L. (2018). Social learning strategies: Bridge-building between fields. Trends in cognitive sciences, 22(7), 651-665.
Laland, K. N., & Hoppitt, W. (2003). Do animals have culture?. Evolutionary Anthropology: Issues, News, and Reviews: Issues, News, and Reviews, 12(3), 150-159.
Laland, K., & Evans, C. (2017). Animal social learning, culture, and tradition. In J. Call, G. M. Burghardt, I. M. Pepperberg, C. T. Snowdon, & T. Zentall (Eds.), APA handbooks in psychology. APA handbook of comparative psychology: Perception, learning, and cognition (pp. 441-460). Washington, DC, US: American Psychological Association.
Lamon, N., Neumann, C., Gruber, T., & Zuberbühler, K. (2017). Kin-based cultural transmission of tool use in wild chimpanzees. Science Advances, 3(4), e1602750.
Marshall-Pescini, S., & Whiten, A. (2008). Chimpanzees (Pan troglodytes) and the question of cumulative culture: an experimental approach. Animal cognition, 11(3), 449-456.
McGrew, W. C. (1974). Tool use by wild chimpanzees in feeding upon driver ants. Journal of Human Evolution, 3(6), 501-508.
McGrew, W. C., & Tutin, C. E. (1978). Evidence for a social custom in wild chimpanzees?. Man, 234-251.
McGrew, W. C., & McGrew, W. C. (1992). Chimpanzee material culture: implications for human evolution. Cambridge University Press.
McGrew, W. C., Marchant, L. F., Scott, S. E., & Tutin, C. E. (2001). Intergroup differences in a social custom of wild chimpanzees: the grooming hand-clasp of the Mahale Mountains. Current Anthropology, 42(1), 148-153.
Möbius, Y., Boesch, C., Koops, K., Matsuzawa, T., & Humle, T. (2008). Cultural differences in army ant predation by West African chimpanzees? A comparative study of microecological variables. Animal Behaviour, 76(1), 37-45.
Morin, O. (2015). How traditions live and die. Oxford University Press.
Nakamura, M., & Uehara, S. (2004). Proximate factors of different types of grooming hand-clasp in Mahale chimpanzees: implications for chimpanzee social customs. Current Anthropology, 45(1), 108-114.
Nishida, T. (1973). The ant-gathering behaviour by the use of tools among wild chimpanzees of the Mahali Mountains. Journal of Human Evolution, 2(5), 357-370.
Kitahara-Frisch, J., & Norikoshi, K. (1982). Spontaneous sponge-making in captive chimpanzees. Journal of Human Evolution, 11(1), 41-47.
Sasaki, T., & Biro, D. (2017). Cumulative culture can emerge from collective intelligence in animal groups. Nature communications, 8(1), 1-6.
Schöning, C., Humle, T., Möbius, Y., & McGrew, W. C. (2008). The nature of culture: technological variation in chimpanzee predation on army ants revisited. Journal of Human Evolution, 55(1), 48-59.
Schuppli, C., & van Schaik, C. P. (2019). Animal cultures: How we've only seen the tip of the iceberg. Evolutionary Human Sciences, 1.
Scott‐Phillips, T., Blancke, S., & Heintz, C. (2018). Four misunderstandings about cultural attraction. Evolutionary Anthropology: Issues, News, and Reviews, 27(4), 162-173.
Sperber, D. (1985). On anthropological knowledge. Cambridge University Press, Cambridge.
Sperber, D., & Claidière, N. (2008). Defining and explaining culture (comments on Richerson and Boyd, Not by genes alone). Biology & Philosophy, 23(2), 283-292.
Sperber, D. (1996). Explaining culture: A naturalistic approach. Cambridge, MA: Cambridge.
Tennie, C., Call, J., & Tomasello, M. (2009). Ratcheting up the ratchet: on the evolution of cumulative culture. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2405-2415.
Tomasello, M., Davis-Dasilva, M., Camak, L., & Bard, K. (1987). Observational learning of tool-use by young chimpanzees. Human evolution, 2(2), 175-183.
Tomasello, M., 1990. Cultural transmission in the tool use and communicatory signaling of chimpanzees. In "Language" and Intelligence in Monkeys and Apes. Comparative and Developmental Perspectives. S.T. Parker & R.K. Gibson (Eds.) Cambridge, Cambridge University Press.
Tomasello, M. (1996). Do apes ape. Social learning in animals: The roots of culture, 319-346.
Tomasello, M., & Call, J. (1997). Primate cognition. Oxford University Press.
Van Leeuwen, E. J., Cronin, K. A., Haun, D. B., Mundry, R., & Bodamer, M. D. (2012). Neighbouring chimpanzee communities show different preferences in social grooming behaviour. Proceedings of the Royal Society B: Biological Sciences, 279(1746), 4362-4367.
Whitehead, H., Laland, K. N., Rendell, L., Thorogood, R., & Whiten, A. (2019). The reach of gene–culture coevolution in animals. Nature communications, 10(1), 1-10.
Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., ... & Boesch, C. (1999). Cultures in chimpanzees. Nature, 399(6737), 682-685.
Whiten, A., Horner, V., Litchfield, C. A., & Marshall-Pescini, S. (2004). How do apes ape?. Animal Learning & Behavior, 32(1), 36-52.
Whiten, A., Horner, V., & De Waal, F. B. (2005). Conformity to cultural norms of tool use in chimpanzees. Nature, 437(7059), 737-740.
Whiten, A., McGuigan, N., Marshall-Pescini, S., & Hopper, L. M. (2009). Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2417-2428.
Whiten, A., & van de Waal, E. (2017). Social learning, culture and the ‘socio-cultural brain’ of human and non-human primates. Neuroscience & Biobehavioral Reviews, 82, 58-75.
Whiten, A. (2019). Cultural evolution in animals. Annual Review of Ecology, Evolution, and Systematics, 50, 27-48.
Yamakoshi G. 2001. Ecology of tool use in wild chimpanzees: toward reconstruction of early hominid evolution. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Tokyo, Japan: Springer Verlag. p 537–556



Dan Sperber 16 October 2020 (01:52)
A composite toolkit or a genuinely unified approach?
Thanks Sadie, Manon, and Josep for this very rich and challenging contribution. There are many points, some more substantial, others merely terminological that I would like to see discussed, but here I will just comment on a very general point. I fully agree with your suggestion that the social learning and the cultural attraction perspectives are both relevant to understanding cultural evolution and should be unified. This integration might take different forms. It might be a matter of drawing on the resources developed within these two approaches: the much more considerable and formally sophisticated work done within the social learning approach and the more sparse but nevertheless, it turns out, insightful work done within the CAT approach; in other terms, fill a toolbox with tools borrowed from both approaches. From a theoretical point of view, this amount to saying that each approach is partially right but misses or denies what the other highlights. Actually, most practitioners of both approaches now agree that they are compatible and in many respects complementary.
One might be more ambitious and aim not just at using a composite toolbox but at developing a unified theoretical framework. That is, instead of viewing faithful copying and cultural attraction as two competing theories, one might agree that both throw light on types of causal processes that help explain the relative stability of some types of behaviours, mental states, and artefacts produced in a population over time, in other terms, of “culture”. Of course, social learning resulting in faithful copying, when it occurs, helps explain this stability. To the extent that it does not provide a full explanation (even under a sensible idealisation of what there is to explain), the social learning approach must indeed be complemented by the study of other types of processes that may also contribute to cultural stability.
CAT has been interested in the heterogenous range of processes that can cause a distribution of behaviours etc at a given time step (for instance, a generational step) to result in a similar distribution at the next time step, with or without fidelity of copying involved. The point has never been to deny that, formally speaking, copying processes could achieve this result or that they actually do contribute to such a result, but to show that, formally speaking, other types of processes could also achieve this result and that they actually do contribute to such a result. Copying processes in CAT are described as homo-attraction, and non-copying processes also contributing to stability are described as hetero-attraction. Most of the literature discussing CAT tends to equate attraction with hetero-attraction, which is a misunderstanding of the approach.
So, in a sense, CAT does suggest a unifying framework of the kind you seem to be hinting at. But, at this stage, this is more a suggestion than a theory. A formal unification has been just waved at through toy formalism. People working in the social learning perspective have, on the other hand, developed sophisticated formal models and tools and they are uniquely well placed to contribute to a meaningful formal unification, if they care to do so.
No less important, from my point of view, is empirical work on the evolution of specific cultural phenomena that directly throws light on the variety and interaction of the processes involved in cultural stability and change (well-illustrated, for instance, in the work of one of the contributor to this project, Helena Miton). Empirical work on animal culture of the kind you discuss would, I believe, greatly contribute to a broadening and deepening of our theoretical perspective.
Dietrich Stout 22 October 2020 (22:09)
Inclusive Cultural Inheritance?
Hi, first I’d like to apologize that I have been unable to participate in these discussions as I’d like to. Afraid the remote teaching has put me over the edge this semester.
Second, I’ll post this comment here but I think it is also relevant to an exchange between Dan and Valentine in the Week 5 thread.
Reading these has made me think of something that puzzles me. Often it seems that advocates of and Extended Evolutionary Synthesis in Biology want to include culture as part of inclusive inheritance but then when they turn to cultural inheritance they model it on the very same gene-centric Modern Synthesis conceptions that they are critiquing as insufficiently inclusive. I must admit that I am not well versed I CAT (hoping to learn!) but could it point the way toward a view of Inclusive Cultural Inheritance?
This relates to some thoughts (reservations) I’ve been having about the whole Cumulative Cultural Evolution (CCE) concept. I’ve now used the term in the title of a few papers, but am starting to think this was a mistake.
As far as I can tell, CCE originated as part of a broader attempt to explain human cognitive uniqueness as a product of enhanced social learning mechanisms including imitation, instruction, and collaboration [1]. High-fidelity reproduction by these mechanisms was proposed to support a cultural evolutionary “ratchet effect” that is cumulative in the specific sense that it generates “sequential improvement over time” [2]. But improvement and progress are dangerous concepts in evolutionary theory [3] and a problematic basis on which to define CCE. For metrics of improvement to reflect anything other than arbitrary preference, they must be grounded on some ultimate criterion such as genetic or cultural fitness [2]. But a well-established term for evolutionary change that increases fitness already exists: adaptation [4]. Persistent trends in human cultural evolution, such as increases in the upper bounds of observed technological complexity [5], do not require an additional mechanism or modality of evolution. As in biological evolution, these are simply expected to occur when selection pressures are constant, trait values are bounded, and/or evolutionary change is biased by developmental or structural factors [6]. Technological evolution may be particularly liable to meet these criteria, insofar as material goals (e.g. subsistence), constraints (e.g. physics), and typical solutions (e.g. adding components or processes) appear relatively stable over time [7]. It is thus unclear that use of the term “cumulative” [e.g. 8] adds anything to the study of cultural evolution apart from implicit progressivist assumptions.
From this perspective, the complexity of human technology is not attributable to the appearance of a single, species-typical cumulative culture capacity but rather reflects an expansion of the range of cultural traits that can evolve at all. If a particular trait cannot be invented and inherited, whether due to limitations on social [9] and/or individual [10, 11] learning, it cannot evolve in any direction. Rather than trying to identify the timing and context for crossing of a CCE Rubicon after which the march of progress dominated, we should focus our efforts on understanding the complex and contingent evolutionary processes [8, 12] by which the cultural evolution of particular technological practices became possible. This would retain the existing focus on social learning mechanisms while expanding the scope of inquiry to consider the broader technological niche [8] including inheritance of material infrastructure [13] and social arrangements [7, 14] as well as interactions between technologies [15]. In other words, we need to consider cultural evolutionary analogies, not only with genetic transmission, but with other aspects of inclusive inheritance (constructive development, reciprocal causation, environmental inheritance)?
So, I ask, how well or poorly do these thoughts align with CAT and its application to animal cultures? Also, should I stop talking about CCE or did I miss something in my attempt at critique?
1. Tomasello, M. et al. (1993) Cultural learning. Behavioral and Brain Sciences 16, 495-552.
2. Mesoudi, A. and Thornton, A. (2018) What is cumulative cultural evolution? Proceedings of the Royal Society B: Biological Sciences 285 (1880), 20180712.
3. Ruse, M. (1996) Monad to man : the concept of progress in evolutionary biology, Harvard University Press.
4. Reeve, H.K. and Sherman, P.W. (1993) Adaptation and the Goals of Evolutionary Research. The Quarterly Review of Biology 68 (1), 1-32.
5. Stout, D. (2011) Stone toolmaking and the evolution of human culture and cognition. Philosophical Transactions of the Royal Society B: Biological Sciences 366 (1567), 1050-1059.
6. McShea, D.W. (1994) Mechanisms of large‐scale evolutionary trends. Evolution 48 (6), 1747-1763.
7. Derex, M. and Mesoudi, A. (2020) Cumulative Cultural Evolution within Evolving Population Structures. Trends in Cognitive Sciences.
8. Stout, D. and Hecht, E.E. (2017) Evolutionary neuroscience of cumulative culture. Proceedings of the National Academy of Sciences 114 (30), 7861-7868.
9. Tennie, C. et al. (2009) Ratcheting up the ratchet: on the evolution of cumulative culture. Philosophical Transactions of the Royal Society B: Biological Sciences 364 (1528), 2405-2415.
10. Whiten, A. et al. (2003) Cultural panthropology. Evolutionary Anthropology: Issues, News, and Reviews 12 (2), 92-105.
11. Osiurak, F. and Reynaud, E. (2019) The Elephant in the Room: What Matters Cognitively in Cumulative Technological Culture. Behavioral and Brain Sciences, 1-57.
12. Stout, D. (2018) Human evolution: history or science? In Rethinking Human Evolution (Schwartz, J.H. ed), pp. 297-317, MIT Press.
13. Pradhan, G.R. et al. (2012) Social organization and the evolution of cumulative technology in apes and hominins. Journal of human evolution 63 (1), 180-190.
14. Powers, S.T. et al. (2016) How institutions shaped the last major evolutionary transition to large-scale human societies. Philosophical Transactions of the Royal Society B: Biological Sciences 371 (1687), 20150098.
15. Kolodny, O. et al. (2015) Evolution in leaps: The punctuated accumulation and loss of cultural innovations. Proceedings of the National Academy of Sciences 112 (49), E6762-E6769.
Mathieu Charbonneau 2 November 2020 (17:23)
The method of exclusion and the grains of description of cultural variation
Thank you Sadie, Manon, and Josep for this exciting early draft.
One element of your draft that I found striking is your discussion of what I understand to be the transmission and stabilization of cultural variation of a same cultural item at different grains of description, and how failing to account for these grains of description can lead the ‘method of exclusion’ used for chimpanzee cultural traditions to erroneously dismiss cultural traditions. By grain of description I mean the classes of variation used to describe a cultural item. One could talk of presence/absence traits: either the population does ant fishing using a stick, either it doesn’t. Such coarse grain of description can be refined by adopting a more precise class of possible variants. For those chimpanzees fishing ants with sticks (the presence trait), they can do so with a long or short stick, and wipe the ants with their free hand or directly with their mouth (here leading to a finer, 2x2 variation space for the presence of ant fishing with a stick). I take your distinction between function/material means/actions to offer just that: adopt a finer grain of description than what is typically used when applying the ‘method of exclusion’.
What is key here is that different processes—social learning mechanisms (homo-attraction), individual/social/ecological factors (of hetero-attraction; see Dan’s comment), etc.—can explain how a same cultural item is passed on and stabilized through time, but do so at different grains of description. For instance, chimpanzees may acquire the trait of fishing ants with sticks from others through local enhancement or emulation, which leads to the high-fidelity transmission (episodic fidelity) of the ‘ant fishing’ technique when coarsely described as a presence/absence trait. At this coarse grain of analysis, these mechanisms are of high-fidelity (propensity fidelity). However, the finer variants of the action (use free hand or mouth) and material means (use short or long stick) may fail to be transmitted by such social learning mechanisms; they would be of low-fidelity (propensity fidelity) for finer grained variation, even if that variation is re-produced relatively uniformly in the population (rigidity/episodic fidelity). (In contrast, imitation would be able to transmit the cultural item at a coarse grain of description and at a finer level, specifically in terms of the variant actions the item can display). What you suggest, instead, is that ecological factors (e.g., ant aggressivity) seems to lead to the stabilization and re-production of the finer variants of stick-length and action (hetero-attraction).
I thus understand your criticism of the ‘method of exclusion’—or reformulate it— as arguing that showing that the stability of a variant behaviour described at a fine grain—e.g., ant fishing with a short stick—can be explained by ecological factors does not preclude that the same cultural item, described at a coarser grain—e.g., transmission of ant fishing behaviour more broadly—, can be transmitted through social learning. The conceptual error of the ‘method of exclusion’ is to conflate these grains of description, and thus misunderstand how different processes and mechanisms of social learning and attraction can act (and act differently) at different grains of description.
My question is: would you agree with this interpretation of your argument regarding the ‘method of exclusion’?
Sadie Tenpas 20 November 2020 (18:25)
Response to Dan
Thank you very much, Dan, for your insightful commentary. We enjoyed thinking over these ideas and integrating them in our revised chapter. Below, we discuss each of your ideas in more detail.
To begin, we would like to address the two approaches toward a unified conceptualization. We agree that both theories, i.e. social learning and cultural attraction, offer complementary insights to understand the causal processes supporting the stability of cultural traditions. We also agree that the toolbox and the unified theoretical approach make fair attempts to bring these theories together. While the toolbox approach highlights what each theory denies (or perhaps rather deemphasizes), we think the toolbox approach treats both theories as complementary. We think it is beneficial to identify and utilize tools within both theoretical frameworks to provide a more holistic, broad approach with greater explanatory power. It may not be necessary to create a wholly new unified theory if there are functional components that already exist, or suggestions allowing room for further integration as you suggest CAT includes. In such, perhaps the toolbox is a good starting point. In regard to your discussion on the position of CAT, we are very intrigued by the notion of hetero- and homo-attraction processes and agree that this framework in combination with ‘tools’ from the social learning approach may be a place to further elaborate our unifying concept.
On your suggestion that both theories recognize one another and consider them to be compatible, if not complementary, we would be hesitant to agree, at least from the social learning perspective. We conducted a brief literature search in leading journals of our fields (Animal Behaviour, Animal Cognition, Journal of Comparative Psychology, American Journal of Primatology, Behavioral Ecology and Sociobiology, Behavioral Ecology) and found no mention of CAT. It is our experience that many of our colleagues are rather unaware of CAT, though further, those who do know of it may view it as a competing theory to social learning, not unsimilar to the Zone of Latent Solutions (ZLS; Tennie et al., 2009). As you stated, though the point of CAT may never have been to deny that copying processes could achieve high-fidelity transmission, we think it is not unfair to say that the role of social learning, either in and of itself or as contributing to other processes, has received little attention by CAT in favor of emphasizing the role of other processes which, as you said, are capable of achieving a similar distribution without copying fidelity. This perspective, like ZLS, challenges the role of social learning, which is helpful in our attempt to define its contribution toward cultural fidelity, but may frame it as ‘competitive’ within the social learning approach.
One thought we had while reading your commentary was on the seemingly dominant perspective within CAT on the role of social learning as faithful copying. It is true that high-fidelity social learning mechanisms such as imitation and teaching are capable of reconstructing products or behaviors or mental states very similar to that of those they learned from (potentially by itself or in combination with other processes). However, the contribution of social learning to cultural fidelity extends to the low-fidelity mechanisms as well, such as local or stimulus enhancement, and we believe this fact may be understated by both theoretical perspectives. Both the social learning perspective and CAT are interested in understanding where cultural fidelity comes from. For the social learning perspective, the answer is predominantly sought by looking to high-fidelity social learning mechanisms and for CAT, the answer is predominantly sought by looking to processes of hetero-attraction. However, a third option may be being overlooked by both approaches, that is, the combination of low-fidelity social learning mechanisms with hetero-attraction processes. We can relate this back to our concept of ‘poles’ in that for any given cultural trait, we expect to always observe some influence by each pole, but depending on the context, the intensity of influence will differ.
Finally, we agree that further detailing the empirical studies on the evolution of specific cultural phenomena is vital to enriching our concept and perspective. We will do so in our revised chapters by expanding upon the examples of ant-dipping, moss-sponging, and hand-clasp grooming through the lens of the discussion in the above paragraph.
Tennie, C., Call, J., & Tomasello, M. (2009). Ratcheting up the ratchet: on the evolution of cumulative culture. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2405-2415.
Sadie Tenpas 20 November 2020 (18:38)
Response to Dietrich
Thank you very much, Dietrich, for your intriguing discussion. We thought you raised many interesting questions, and we will answer two as best as we can. However, we feel that some may be more satisfactorily addressed by other, more qualified, members of the project and we would encourage that we continue this discussion further at our later conference.
First, on your point that improvement and progress are dangerous concepts in evolutionary theory, we agree that these concepts must be used carefully. If we understand your position correctly, you suggest that for improvement to be evolutionarily significant, it must be grounded by fitness benefits, which we agree. Further, you propose that the trends observed in the evolution of human culture, like the increases in the upper bounds of observed technological complexity, do not require additional modalities or mechanisms of evolution beyond adaptation to occur. In terms of requiring additional modalities or mechanisms of evolution to observe the increase in technological complexity, we would agree that this is not necessary, especially if technological complexity can cumulate through low-fidelity social learning mechanisms. Take for example the creation of brush-tip tools for termite fishing in chimpanzees (Sanz et al., 2004). Chimpanzees use sticks with frayed ends to collect termites. The process of acquiring this additional component (i.e. the frayed end) may have simply occurred because one individual used the stick to the extent that it had frayed, then another found the stick and emulated what they observed, recreating the tool. In this scenario, the improvement results in a more complex tool, but not by any specialized, high-fidelity learning mechanisms. Further, improvement may be defined on different levels depending on context, in some cases changes toward simplicity may be better and this is often the case in evolution (e.g. parasite digestive tracts). In this sense, we can understand improvement as the selection for efficiency of the end product on which evolution acts on. We think improvement and complexity are thus not necessarily the same.
Second, we don’t think that the term “cumulative” doesn’t add anything to the study of cultural evolution as it describes the nature by which changes in cultural traits can accumulate beyond the capacity of a single individual, and this does not necessarily require enhanced social learning mechanisms.
Sanz, C., Morgan, D., & Gulick, S. (2004). New insights into chimpanzees, tools, and termites from the Congo Basin. The American Naturalist, 164(5), 567-581.
Sarah Michelle Pope 3 December 2020 (22:06)
Disentangling individual vs group (in)flexibility when (un)necessary.
Thank you Sadie, Manon, and Josep - this was a really interesting read. Apologies for the delayed comments!
There are two themes that emerged from my notes when reading this early draft. The first, is that I really appreciate the extent to which you have delved into the complexity of analyzing inflexible vs flexible behavior. I especially liked your use of ‘poles’ to conceptualize the various influences on cultural traits and how they might work together to shape traits’ inherent flexibility.
Second, I want to try and understand Figure 1 a bit more. Let me preface this by saying that my chapter (Week 8) is a terminological nightmare. So hopefully this discussion can be mutually beneficial!
I’m wondering why you made the decision to separate (in)flexibility that occurs at the group level vs the individual level. Isn’t group (in)flexibility just a compilation of individuals’ (in)flexibilities? In other words, wouldn’t a flexible group contain majority ‘liberal’ individuals? Or at least influential ones… I guess I’m confused about why groups and individuals need different terms to describe more or less the same contexts [(in)flexibility when (un)necessary].
My other questions are geared at understanding why you chose these specific terms. For instance, the use of ‘innovation’ to describe what my chapter refers to as responsive flexibility (switching or searching that occurs when the current strategy no longer works). My conceptualization of innovation is that it occurs when a novel strategy is enacted – but I think that necessary strategy switching can also occur without involving a new approach. For instance, in many places it is necessary to switch to wearing coats in winter but this is not a novel strategy. I’m also more familiar with 'shifting' and 'fixedness' as terms used to describe individual’s ability/inability to flexibly adjust their behavior, rather than groups’. I think this will be really interesting to discuss at some point because I agree that they are necessary distinctions and I think you’ve done a great job describing the different contexts in which flexibility and inflexibility occur.
Thank you for a really thought provoking chapter. I’m looking forward to chatting at some point!
Best,
Sarah
Sadie Tenpas 7 December 2020 (12:00)
Response to Mathieu
Thank you very much, Mathieu, for your thoughtful interpretation and discussion. We believe your reformulation of our argument using fine and coarse grains highlights an important point: these grains refer to different aspects of the observed behavior. However, we would conceptualize them differently. You considered the individual/social/ecological factors, as we describe them in the chapter, as hetero-attraction and social learning mechanisms as homo-attraction. Our proposal, however, is that the social factors include social learning mechanisms. It is important to our argument to make this clear because we believe the individual/social/ecological factors are interactive and non-hierarchical. When considering this, your reformulation of our position using fine and coarse grains no longer fits our original proposal. Even so, we appreciate your reinterpretation of our argument as it has created a lens through which we discovered more about our position as we explain below.
While we agree that our distinction between function, material means, and actions offers a finer grain of description than what is typically used when applying the method of exclusion (MoE), this, as applied in your reformulation, doesn’t fully capture our intention when examining these features. Instead of conceptualizing the grains in terms of the descriptions of the observed cultural variants (coarse: presence/absence vs fine: function/material means/actions), we prefer to conceptualize the different grains in terms of which aspect of a particular trait a naïve chimpanzee picks up. Thus, the fine grain refers to how a cultural trait is performed, its means of its enactment. In contrast, the coarse grain refers to why a cultural trait is performed, the goal motivating the behavior or the ends produced by its enactment. In this case, the naïve observer copies from a model displaying the trait, imitation and emulation would be the social learning mechanisms responsible for the acquisition of means and ends (or goals), respectively.
From our perspective, the stability of a behavior would result from some combination of individual/social/ecological factors (your fine grains), rather than hetero-attraction at the fine grain and homo-attraction at the coarse grain, as you seem to suggest. We think that the combination of the individual/social/ecological factors creates a larger dynamic structure of influence on the cultural behavior. When social learning (e.g., imitation or emulation) contribute to the acquisition of the trait, it is justified to speak of cultural behavior. Moreover, even low-fidelity social learning mechanisms would be sufficient for transmission provided individual and ecological cultural attractors serve to stabilize it. Therefore, we agree with your concluding statement that showing that the stability of a variant behavior can be explained, in part, by ecological factors does not preclude that the same cultural item can be transmitted through social learning, and that this is a conceptual problem for the MoE. However, our main criticism of the MoE is that it ignores how the individual/social/ecological factors can function together, resulting in high-fidelity transmission (episodic fidelity) that doesn’t rely on social learning mechanisms alone, especially high-fidelity transmission mechanisms (propensity fidelity) like imitation.
Sadie Tenpas 27 January 2021 (12:30)
Response to Sarah
Thank you very much Sarah for your thoughtful questions and kind comments. We were glad to see that you found our illustration with poles helpful!
Regarding your first question, as to why we distinguish between (in)flexibility at the individual and group level, we have done so because we think the group is more than the sum of individuals. For example, being flexible or not is most likely not an all or nothing response. Individuals react to certain contexts. In particular, social dynamics, like majorities or immigrating to a new group, can influence an individual and a group’s (in)flexible behavior quite differently. As you hinted at, there may be particularly influential individuals that when observed innovating, might initiate a change in group behavior if they are biased by such a social learning strategy. However, it has often been described that within groups of chimpanzees, lower ranking, or otherwise less influential, individuals tend to innovate more frequently, but those innovations are less frequently transmitted and maintained by the group. Further, we know that chimpanzees demonstrate flexible behavior in a wide array of domains and environmental settings, yet they have also demonstrated surprisingly ‘conservative’ behavior by sticking to pre-existing traditions, despite innovations within a group. Therefore, we find it important to distinguish between individual and group (in)flexibility to investigate these differences.
In addressing your questions related to the terms used in Figure 1, we’d like to clarify that this thought experiment was meant to help us explore the different manifestations of (in)flexible behavior across different levels and in doing so we applied commonly used terms in our field to define those situations. We did not intend to present these terms necessarily as new definitions, but rather we found them to be most fitting for the distinctions we made. That said, we are looking forward to a discussion with all of the contributors on these terms and how we might use them consistently throughout the book, as well as hearing other’s thoughts on why they might prefer one term over another.
We hope we have offered some clarifications to our intentions of differentiating between individual and group-levels and of using specific terms - we look forward to discussing more with you in the future!